Some background
Spermidine is a naturally occurring polyamine, alongside other compounds like spermine and putrescine. It is involved in various biological processes and plays essential roles in cell growth, proliferation, and differentiation.
Spermidine has a host of effects in the body, ranging from anti-inflammatory properties and antioxidant functions via arginine bioavailability and nitric oxide (NO) production to enhancement of mitochondrial metabolic function and respiration and improved protein homeostasis. More importantly however, it has been primarily studied in relationship to autophagy, a cellular process responsible for the degradation and recycling of cellular components. Similar to fasting, spermidine appears to promote autophagy, which is important for cellular homeostasis and the removal of damaged or unnecessary cellular components.
Spermidine can come from 3 complementary routes in the body:
- through endogenous production in our cells (see below)
- through production by our gut microbiome
- through our diet, via foods such as wheat germ, (fermented) soy beans such as natto, mature cheese, mushrooms, peas, nuts, apples & pears, or broccoli. Average daily nutritional intake of spermidine varies between 7 and 25 mg, with the highest amounts encountered in the Mediterranean diet.

Aging primer
Aging is a complex, multifaceted biological process characterized by a gradual and progressive decline in physiological function, resulting in an increased vulnerability to various diseases and an increased risk of mortality over time. It is a universal and intrinsic aspect of life, affecting all living organisms to some extent. Aging involves a combination of genetic, environmental, and stochastic factors, and it manifests at various levels, including molecular, cellular, tissue, and organismal levels. It is characterized by a host of factors, also known as the hallmarks of aging, to which we added an extra one at the end:
- genomic instability: tendency of a cell’s genetic material, particularly DNA, to undergo structural alterations, mutations, or changes, increasing the risk of errors in genetic information
- telomere attrition: gradual shortening of the protective caps at the ends of chromosomes, known as telomeres, with each cell division
- epigenetic alterations: changes in gene expression patterns, via methylation or acetylation, that occur without alterations to the underlying DNA sequence
- loss of proteostasis: disruption of the balanced regulation of protein synthesis, folding, and degradation in cells, leading to the accumulation of misfolded or damaged proteins
- mitochondrial dysfunction: impaired or suboptimal functioning of mitochondria, the cellular organelles responsible for energy production
- cellular senescence: state of irreversible growth arrest in which cells cease to divide in an attempt to repair damaged tissue and prevent potential cancerous transformations
- stem cell exhaustion: reduced regenerative capacity and compromised tissue repair, due to a depletion or functional decline of the pool of stem cells within a tissue or organ
- altered intercellular communication: changes in signaling pathways and communication between cells
- deregulated nutrient sensing: disruptions in the proper perception and response to nutrient levels within cells, often associated with metabolic disorders, such as diabetes
- chronic inflammation: prolonged and dysregulated immune response characterized by sustained activation of inflammatory pathways.
Through its effects on mitochondrial function, protein homeostasis, inflammation, but mostly on autophagy, it can be argued that spermidine is a useful nutrient tool in any anti aging portfolio.

With aging, diabetes, cardiovascular disease, cancer, and neurodegeneration become ever more prevalent. As human lifespan increases worldwide, these 4 horsemen of death, as described by Peter Attia in his book Outlive, are the main conditions causing death in the vast majority of people. This is why, once genetically and metabolically balanced, focusing on delaying the arrival of these conditions should be an ongoing focus in any attempt of improving wellbeing, longevity and healthspan, as we do through our Centenarian Maintenance approach.
Spermidine Biochemistry
We mentioned that endogenous spermidine production in our cells is one the 3 complementary routes contributing to the overall spermidine pool. And because a picture is worth a thousand words as they say, here is a view on the polyamine transport, biosynthesis & catabolism from the excellent review „Spermidine in health and disease”:
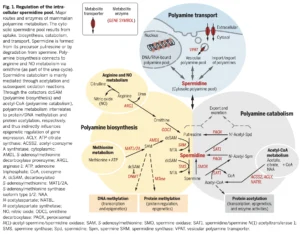
In the middle of the picture, you can see that spermidine is formed from its precursor putrescine or by degradation from spermine, its other 2 polyamine cousins. What is also noteworthy to mention is the connection to two other key amino acids and namely:
- methionine, via the co-factor dcSAM (decarboxylated S-adenosylmethionine)
- arginine and NO metabolism, via ornithine (as part of the urea cycle). This is probably the main mechanism through which spermidine can impact cardiovascular function through NO-induced vasodilation
How Spermidine works
Cellular and molecular mechanisms of spermidine-mediated health protection are all related more or less to autophagy:
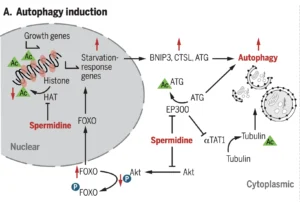
At a mechanistic level, autophagy induction by spermidine works on two fronts:
- through inhibition of the acetyl transferase EP300, primarily resulting in deacetylation of ATG and the converse acetylation of Tubulin, both of which induce autophagy
- through the regulation of the FOXO3 transcription factor, via inhibition of Akt phosphorylation, as well as through the inhibition of HAT (histone acetyl transferase), both resulting in autophagy induction via BNIP3, CTSL and again ATG.
Moreover, spermidine can generally cause mTORC1 inhibition and activate phosphorylation of AMPK, both of which induce autophagy. This effect can be enhanced by combining spermidine with resveratrol.
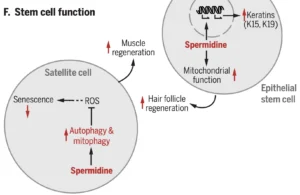
One of the areas depending on spermidine-triggered autophagy is the suppression of stem cell senescence in satellite cells, whereas spermidine promotes mitochondrial function and production of keratins in epithelial stem cells, independent of autophagy. These ensure both muscle and hair follicle regeneration:
Some cautionary notes
Whereas spermidine has a host of beneficial effects that are worth exploiting nutritionally and/or supplementally, there are possible contraindications, especially for patients with advanced cancer and renal failure conditions. Several pathologies have been associated with increased polyamine concentrations, reason for which polyamines in general could also be used as disease biomarkers in conditions like cancer, neurodegenerative disease, stroke, and renal failure.
This is why, supplementation in general works best in optimal health conditions, before disease sets in. Once that line has been crossed, a more pharmacological approach is best to be employed. However, a balanced, healthy diet, of the sort we offer at our Health Hub at OCLU, ensures optimal nutrient levels, including that of spermidine, so fortunately you won’t cross that line. Ever.