Autophagy is a vital cellular process that plays a crucial role in maintaining our bodies’ health and functionality. It is a mechanism through which cells can recycle their own damaged and unnecessary cell components, including proteins, organelles, and even complete cells. Every cell in the human body can run autophagy, and it is essential for various physiological processes, including development, immune system performance and stress response (Parzych & Klionsky, 2014).
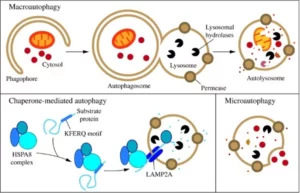
There are three main types of autophagy: Macroautophagy, Microautophagy and Chaperone-mediated autophagy. They all have in common the fact that all three culminate in the delivery of a cellular cargo to the lysosome (a cell organelle with hydrolytic enzymes) for degradation and recycling. We will explain in more detail each one of them and its relevance for your health.
Macroautophagy
Macroautophagy relies on de novo formation of cytosolic double-membrane vesicles, aka autophagosomes, to sequester and transport cargo to the lysosome. This type of autophagy is the most well-studied and is also called autophagosome-mediated autophagy or simply autophagy.

Autophagy involves different stages: initiation, elongation, and completion of the autophagosome. In mammals, autophagosome generation is initiated at multiple sites throughout the cytoplasm. The initiation starts thanks to the activation of autophagy-related proteins (ATGs). Then, during elongation the membrane begins to expand, giving place to the Phagophore. Lastly, as the phagophore expands, the membrane bends to ultimately generate a spherical autophagosome, which is transported to a lysosome with which it fuses, and thus the necessary cellular material is degraded (Parzych & Klionsky, 2014).
Autophagy is induced or enhanced by a wide range of extra- and intracellular stresses including nutrient starvation (fasting or caloric restriction), the presence or absence of insulin and other growth factors, hypoxia, and Endoplasmic Reticulum stress. Also, there are certain drugs and compounds that induce autophagy, such as rapamycin and curcumin (found in turmeric).
But how exactly is autophagy induced?
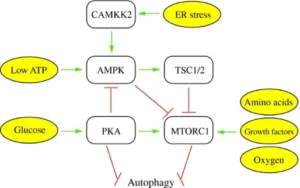
There are two main pathways involved in nutrient availability that inhibit or suppress autophagy: cAMP-dependent protein kinase A (PKA) and mTOR (mechanistic target of rapamycin). High levels of nutrients, such as glucose and amino acids, and certain signaling pathways for anabolic processes such as the mTOR pathways, induce cell growth and proliferation, thus suppressing autophagy (Parzych & Klionsky, 2014). As also shown in the diagram below, high availability of oxygen also induces mTOR pathways, so hypoxia (absence of oxygen) could induce autophagy as well.
But ok, you may now be wondering: if autophagy basically involves your cells eating themselves, how this can be beneficial for you? Well, there are several health benefits of autophagy. Imagine there is a cell that is not working properly, and it eats itself to avoid reproduction and causing damage to future cells; this cell is a superhero martir, right?
All cells can do autophagy, and this helps them to maintain their homeostasis, prevent accumulation of damaged or abnormal proteins, and remove unwanted or harmful substances. Autophagy has been shown to have anti-aging effects and it plays a huge role in preventing several age-related diseases, such as neurodegenerative disorders, cancer, and cardiovascular disease. Sounds great, right?
Microautophagy
Microautophagy refers to the process in which cytoplasmic contents enter the lysosome through an invagination or deformation of the lysosomal membrane. Due to the limited number of tools available for the study of this type of autophagy, there is very little information on this process. Its regulation and possible effects on human health and disease are still unknown (Parzych & Klionsky, 2014).
Chaperone-Mediated Autophagy (CMA)
This type of autophagy is highly specific because it involves the recognition and uptake of specific proteins by chaperon proteins. CMA degrades a wide range of substrate proteins, including certain glycolytic enzymes, transcription factors and their inhibitors, calcium and lipid binding proteins, proteasome subunits, and proteins involved in vesicular trafficking. This process is also less well understood than macroautophagy and is believed to play a very specialized role in the cell (Parzych & Klionsky, 2014).
Genetic predispositions
Again… as you may now know, genetic predispositions are a determinant that regulates autophagy processes in each person. As mentioned before, there are some proteins that initiate autophagy processes and namely ATGs; it is estimated that there are more than 69 different ATGs, so SNPs in any of these proteins, can affect autophagy. ATGs include: ULKs (unc-51 like autophagy activation kinase), PRKAAs (protein kinase ARN-activated-like autophagy-inducing kinase, and SIRTs (sirtuins).
Some examples of SNPs in different proteins are shown below, as well as the associated risks for each one.
- ULK1 (rs145451295, rs55815560, rs145279005): associated with schizophrenia.
- ATG7 (rs1375206): associated to Alzheimer’s disease.
- ATG3 (rs35619591): associated to type 2 diabetes.
- ATG5 (rs671116, rs510432): associated with esophageal and liver carcinoma.
- PIK3C3 (rs52911): associated with a Protective effect for esophageal carcinoma.
Pump up your autophagy!
While genetics play a significant role in the regulation of autophagy processes, there are some habits that could influence autophagy, such as environmental factors, lifestyle and nutrition. For example, fasting or caloric restriction can induce autophagy, while high-fat, high-sugar and caloric surplus could inhibit autophagy. Exercise has also been shown to enhance autophagy, while chronic stress inhibits it.
In terms of supplementation, as mentioned before, curcumin could induce autophagy, as well as resveratrol, a compound found in grapes and red wine, known to induce autophagy in cancer cells. Other interesting compounds are quercetin (found in red wine, onions, green tea, apples, etc.), kaempferol (found in broccoli, green tea, etc.) and berberine, among others (Wang et al., 2022).
Don’t forget to contact us at Nutrifix for a free consultation on how you can optimize your genes and metabolism and enhance different beneficial processes, such as autophagy, to live a better, healthy, longer, life!