If you are really serious about longevity, you should honestly consider the information below, based on a practical and more technical approach than the wishy washy pseudo-spiritual arguments around this topic. But first things first, let’s make sure we are clear on our goal a bit more precisely: we are targeting an increased healthspan, not a mere increased lifespan. That is to say, we want to maximize the amount of healthy, functional lifetime primarily; this is the driver for a consequent increased lifespan, out of which the percentage of years when one can function optimally is maxed out.
A bit of aging pseudo-mathematics

Now, healthspan is our y variable in the optimization equation we are putting forth, with the first derivative of that being represented by lifespan. Ideally healthspan equals lifespan though, so that almost no amount of one’s lifetime is spent in any degree of incapacity. Which means the boundaries of both are ideally birth and, yes.. death. On the face of it, this may seem a dark and gloomy approach to consider, and admittedly there’s a ton of spiritual work all of us have to do in our lifetime to come at peace with the prospect of this inevitable reality, but this is also extremely concrete, rigorous and actionable.
So in a nutshell, yes, chanting, sound healing and gratitude all can help with overall health and wellbeing (to rather unmeasurable degrees), but in order to prolong health and lifespan, one essentially needs to postpone death as much as possible. And so.. thinking about the main causes of death and how these can be postponed, or the 4 Horsemen of Death as Peter Attia calls them, makes a lot of practical sense.
A bit of marketing
Metabolic, vascular, neurodegenerative diseases and cancer are the 4 main causes of death worldwide and going after each one as a preventative approach in increasing healthspan by postponing death essentially is what we are aiming to help you achieve through the Centenarian Maintenance package we offer. This builds very well on the Complete Genetic Optimization package where we plug in all the genetic holes (as in predispositions) if you want. But this only ensures optimal functioning. Going a step beyond the regular aging process means setting up more ambitious age-adjusted goals to measure and aim towards, with the help of an arsenal of behaviours, supplements and even medications.

For example, on the cardiovascular (most relevant of the vascular disease pillar) front, aiming at 40 for an LDL (low density lipoprotein) level of a healthy 30 year old, can be regarding as dialling back the aging clock with 10 years from an LDL perspective. Doing the same on multiple fronts for all the 4 horsemen should get you there. This is at least the longevity bet that we, and others, are placing; because, obviously, there is no certainty with pretty much anything in life. But at least, to the very best of our scientific knowledge, this approach gets closest to that.
One thing to note in general, which applies to all that science discovers, is that natural phenomena are not separable into concepts in reality, as we like to conceptualize in our brain. We need that level of categorization, as we do with our 4 horsemen here or with any metabolic pathway we describe, in order to understand a particular concept (e.g. cardiovascular disease) and organize our approach to achieve a specific goal (e.g. lowering LDL) regarding that. But in reality everything is interconnected. This becomes obvious especially when you look at cellular interactions at a molecular level, where certain particularities can trickle up in a host of varied symptoms. So essentially, what we call a cardiovascular issue can have a very similar set of molecular causes as what we would describe as a metabolic symptom, say insulin resistance.

Which brings me to the second point, which is related to a necessity to reframe our approach regarding disease from purely considering symptomatology to also considering the molecular basis of what triggers those perceived symptoms. On one hand symptoms are most often subjectively described and are so interpretable based on the level of consciousness of each subject experiencing those symptoms. On another hand, more importantly, symptoms are manifestations of a conglomerate of molecular disparities. Once you target the latter, the hope of any cure put forward by humanity is that the body can do its magic to realign things. This is how cures work anyway.
Symptoms can’t be targeted, molecular pathways can. So we should start thinking about impaired propensities to clear LDL or about insulin homeostasis, than about the fact that we feel dizzy or have migraines. Of course, this is more technical in nature, but this is what we are aiming to do in our genetic and metabolic optimization approach in general and also with our Centenarian Maintenance aging package in particular, the subject of this article.
Horseman #1: Metabolic Disease
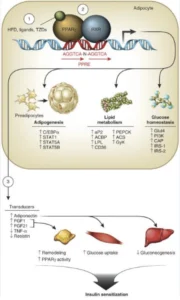
That being said, a healthy metabolism lies at the cornerstone of a disease-free, potentially aging-reversal life. Whether they are associated with obesity, satiety and weight loss, or with lipid transport and metabolism, cell growth and glucose metabolism, ketosis or gut health, there is a host of genes we look at in the metabolic disease front. These range from FTO and LEPR, to ADIPOQ, PPARG, FADSs, PANKs, ACATs, SLC16A1, IRS1, APOA2 and FUT2, among many others. And all have a story worth investigating from an aging perspective, in case of significant genetic variations.
For example, PPARG belongs to the PPAR family, which is part of the steroid/thyroid/ retinoid receptor superfamily and control a variety of genes in several pathways of lipid metabolism, including fatty acid transport, cellular uptake, intracellular binding and activation, as well as catabolism or storage. The PPARG gene is mostly abundant in adipose tissue and is synergistically induced by insulin, IGF1 and corticosteroids. It plays a role in adipogenesis, or the creation of fat tissue and is activated also by high doses of NSAIDs, such as ibuprofen. PPARG also mediates brown adipose tissue increased metabolic effects, alongside the PGC1 co-activator.
Horseman #2: (Cardio)vascular Disease
On the cardiovascular end, there is a battery of relevant genes we look at, mostly related to the Renin-Angiotensin-Aldosterone System (RAAS) and nitric oxide, all key in blood pressure, one of the key risks factors for cardiovascular disease. Outside the habit of smoking, lipoprotein-related issues are another key risk factor for cardiovascular disease, but we categorize that as part of lipid metabolism under the previous horseman.
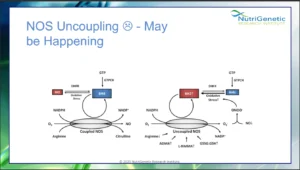
Nitric oxide for example, the target of drugs like Viagra, and involved in both vasodilation, as well as key oxidative processes, is synthesized by the 3 isoforms of Nitric Oxide Synthase, NOS1 (the neural form), NOS2 (the inducible form) and NOS3 (the endothelial form). Normally, NOS enzymes convert oxygen and arginine to nitric oxide and citrulline in the presence of NADPH and BH4.
The endothelial form NOS3 can be upregulated by genes like SIRT1, compounds from foods like rhubarb or herbs like skullcap, whereas it can be uncoupled by compounds like BPA, which is found in plastic. When this happens, the process yield the inflammatory molecule superoxide, instead of nitric oxide; in excess, this starts a nasty inflammatory cascade, ultimately leading to even nastier inflammatory molecules, like peroxynitrite, reason for which NOS3 uncoupling should be addressed. And this is because a vasoconstricted and inflammed endothelium is generally a bad sign on the cardiovascular front. Imagine that!
Horseman #3: Neurodegenerative Disease
Usually a late age concern, ‘losing one’s mind’ can take a host of symptomatologic forms from the ubiquitous Alzheimer’s, to Parkinson’s, Huntington’s and Schizophrenia, to ‘lighter’ conditions such as OCD, ADHD, autism, depression or neuroplasticity issues. Genes like APOE, BDNF, QPRT, DAOA, COMT, GAD, GAMT and CREB1 govern this host of issues and we consider all of them and many more in guiding you through your mind’s game.
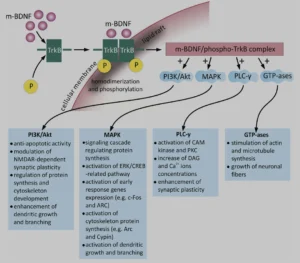
BDNF (brain-derived neurothrophic factor) for example belongs to a family of small secreted proteins that also include nerve growth factor, neurotrophin 3, and neurotrophin 4. Paramount to both Alzheimer’s and neuroplasticity in general, BDNF has a host of roles in the brain. It regulates both neurogenesis and excitatory synapses during early LTP (long term potentiation), via glutamate modulation of the NMDAR (N-methyl-D-aspartate receptors) and consequent cellular calcium influx. And yes, it is as complicated as it sounds. The three main intracellular signaling cascades activated by BDNF are:
- the phosphatidylinositol 3-kinase (PI3K)–Akt pathway,
- the Ras–mitogen-activated protein kinase (MAPK) pathway,
- and the PLCγ–Ca2+ pathway, all with important subsequent metabolic effects, as can be seen in the diagram below:
Horseman #4: Cancer
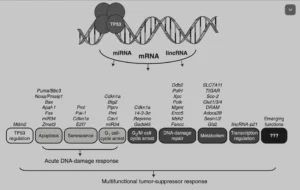
The Emperor of all Maladies, as Siddhartha Mukherjee makes the case for cancer to be, is the most complex of them all. It can be regarded as the immune system out of sync, and significant variations in genes like TP53, KRAS, PIK3CA, BRCA1 and 2, TSC1 and 2, RHEB or RAF1 may definitely play a role.
Of these, TP53, a tumour suppressor protein, has been extensively studied. It is activated after a variety of stresses, such as radiation, by toxic substances, hypoxia, high levels of reactive oxygen species (ROS) or simply put oxidation, uncontrolled cell cycle etc. As a consequence, it can trigger either DNA repair or a host of suppressing processes, such as cell cycle arrest, cellular senescence, apoptosis or autophagy. It does so through a host of downstream genes, such as CDKN1a (p21), GLUT1, 3 and 4, SLC7A11, or ADORA2B:
But, why bother?
Well, first of all, it is important to find out about any genetic predispositions, that is, to measure your genetic baseline. Then you can confirm or not, as the case may be, any metabolic manifestation of those genetic predispositions via additional metabolic testing. Once that is in place, we at least know where and how to act, whether this may be behaviourally or via nutrition, supplements or even medications. Then re-assess via relevant metabolic tests and adjust depending on how you respond to particular interventions. This is how to go about optimizing the aging process.
So do you want to be part of the Centenarian Club that will really be able to appreciate the passing of time, measured in centuries? We’re here for you. At least for some while now :).