Iron: the Ore of Inflammation
Let’s briefly discuss about how iron is related to a couple of inflammatory molecules inside the body, via what is the Fenton reaction, a chemical reaction that takes place within the body and leads to increased inflammation. In a nutshell, it is a process by which excess iron ions take control of hydrogen peroxide (H2O2) to create hydroxyl radicals (OH-), which are extremely reactive and harmful molecules. Numerous things, such as oxidative stress, inflammation, and specific environmental toxins, can start the Fenton reaction.

Superoxide (O2-), a highly reactive molecule produced by the body as a byproduct of regular metabolic processes, is one of the key participants in the Fenton reaction. We learned in the previous article how elevated superoxide can hijack nitric oxide (NO) and give birth to peroxynitrite (ONOO-). Under conditions of oxidative stress and inflammation, superoxide is produced at higher levels and can also aid in the Fenton reaction by acting as a substrate for the OH- synthesis. Let’s see how this happens.
Superoxide distumase (SOD): friend or foe?
To protect the body from the damaging effects of superoxide and the Fenton reaction, the body has several mechanisms in place to neutralize these molecules. One such mechanism is the enzyme superoxide dismutase (SOD), which converts superoxide to hydrogen peroxide (H2O2). However, H2O2 can be hijacked by the Fenton reaction to produce OH-. This occurs when H2O2 reacts with iron ions to produce OH-.
The OH- radicals produced by the Fenton reaction are highly reactive and can cause damage to the cells and tissues in the body, leading to inflammation. OH-, a third generation free radical species (derived from generation I, O2- and generation II, H2O2) is actually the most damaging free radical, having a very fast oxidation reaction time with other molecules.
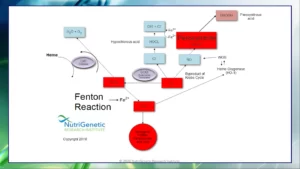
Slowed glutathione and catalase enzymes unable to clear any excess H2O2 can fuel the OH- production process. However, any excess iron in the body adds fuel on this inflammatory fire. Except for any genetic predispositions that may yield increased physiological iron levels, a few lifestyle factors are important to maintain homeostatic iron levels:
- moderate red meat and alcohol consumption, as both tend to increase iron levels in the body
- cooking in non-iron skillets
- avoiding iron synergists, such as vitamin C, that increase its absorption (at least have that apart from iron-rich meal)
Iron genetics & how nutrigenomics can help
There is some evidence to suggest that certain genetic predispositions may increase the risk of Fenton reaction upregulation. For example, certain variations in the HFE gene, and namely in the C282Y, H63D and S65C SNPs, may cause an increased predisposition to an iron overload condition called hereditary hemochromatosis. FTL, is another gene that regulates intracellular iron storage, process that can be upregulated in case of specific FTL SNPs, such as rs398124633. Alternatively FTL can act as an anti-oxidant against the Fenton reaction. Finally, as we will see in a later article, an overactive HMOX gene can also increase the pro-oxidant iron form, Fe2+, thus speeding up the Fenton reaction.
On top of a healthy lifestyle baseline, a few supplements have proven particularly helpful to tame down the highly inflammatory Fenton reaction:
- on one hand rhodiola, vitamin E and catalase are studied for their role in supporting the conversion of H2O2 into H2O and O2
- alternatively, Selenium and Riboflavin (vitamin B2) support the role of Glutathione Peroxidase to turn H2O2 into H2O and O2
- terpenes in clove may reduce iron absorption
- and finally, luteolin in celery, parsley and cruciferous vegetables may suppress the Fenton reaction itself

And just to be on the safe side of inflammation, hydrogen enriched water can help mitigate the extra OH- molecules inevitably generated during sustained metabolic processes.
Now, this is just the beginning of our healthspan optimization approach, but don’t get scared; we can make it super easy for you!